According to the OSHA Combustible Dust National Emphasis Program, “pharmaceutical preparations” are one of the most at-risk industries for combustible dust-related fires and explosions. Pharmaceutical manufacturing ultimately involves the handling and processing of various active pharmaceutical ingredients (APIs) and non-active excipients including fillers, binders, and preservatives. Due to their fine particle size distributions and chemical compositions, many of these ingredients can act as fuel for combustion and present inherent dust fire and explosion hazards.
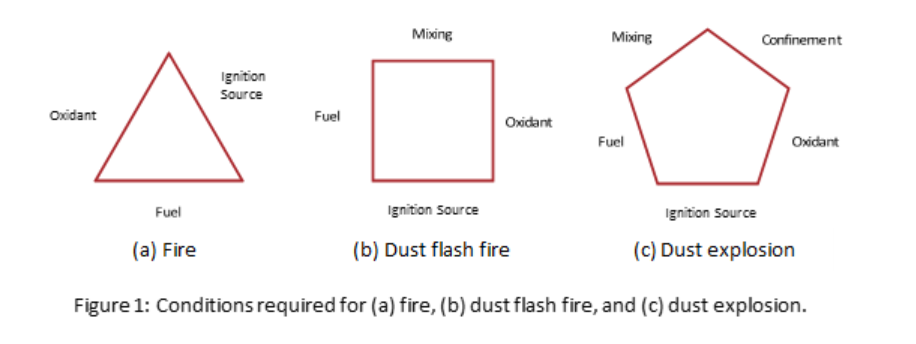
Oral solid dosage (OSD) drug production poses an even greater risk for dust-related fires and explosions due to the large quantities of ingredients handled in powder form. These process operations are known to handle high volumes of combustible ingredients and, as such, present credible dust explosion hazards. Moreover, five conditions required for a dust explosion to occur are readily present in OSD manufacturing processes:
- presence of a combustible dust
- dispersion of dust at a concentration within the explosible range
- presence of an oxidant
- presence of a competent ignition source
- containment of dispersed dust within a confined or semi-confined vessel or area
Quantifying the fire and explosion risk associated with OSD drug manufacturing requires identifying the materials' explosion severity and ignition sensitivity parameters. Most pharmaceutical manufacturing facilities handle a wide variety of APIs and excipients that vary considerably in their chemical and physical compositions and exhibit drastically different explosion severity and ignition sensitivity parameters. Depending on the nature of the process being considered, it is not always practicable to test all materials handled. With the help of a qualified consultant, pharmaceutical manufacturers can establish a testing strategy to narrow down the number of material samples to be tested.
Suppose a material is suspected of being explosible in dust cloud form. In that case, it is highly recommended that it be tested by a qualified laboratory using applicable ASTM testing standards to determine the material-specific explosibility parameters.
These explosibility parameters are often used as key inputs in safe process design and can help design explosion protection systems, implement ignitions source control methods, and identify safe operating procedures. However, because API and excipient ingredient information tends to be proprietary, publicly available explosibility data is limited. Lack of access to data may impede the identification of design parameters for explosion mitigation devices and protective measures, further resulting in various design challenges.
For example, if overly conservative explosibility parameters are selected for the process design, the equipment may be over-designed for handling materials and result in unnecessary capital expenditure. On the other hand, if the chosen explosibility parameters do not encompass the most hazardous materials handled in the process, a catastrophic dust explosion could occur, resulting in facility or equipment damage, process downtime, or severe injury to facility personnel.
Pharmaceutical manufacturers must understand the operational risks of handling combustible APIs and excipients in OSD production. Lack of awareness of potential hazards posed by combustible dusts is one of the root causes of major incidents involving dust explosions. Due to variances in API and excipient chemical and physical compositions, explosibility parameters obtained from literature resources or other manufacturing facilities may not be suitable for the design of explosion protection systems. Material-specific explosibility testing is required to properly determine the most hazardous parameters associated with APIs and excipients and mitigate the risk of combustible dust explosions.
